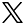We aim to elucidate and to regulate the mechanism of brain aging that leads to the onset of Alzheimer's Disease.


Takaomi Saido, Ph.D.
Team Leader, Proteolytic Neuroscience
takaomi.saido [at] riken.jp
Research Overview
The aim of our research is to understand the mechanism of brain aging with specific emphasis on the study Alzheimer's disease (AD) through proteolysis. Proteolytic reactions often play critical roles in both physiological and pathological circumstances because of their irreversible nature, but their actual in vivo functions particularly in brain are not yet well understood. Among the various aspects of protease involvement in neuropathophysiology, our research focuses on two major themes. One is the metabolism of amyloid-βpeptide (Aβ), the cortical deposition of which triggers the pathological cascade leading to AD. Under physiological conditions, Aβ is constantly produced from its precursor and immediately catabolized, whereas dysmetabolism of Aβ seems to lead to pathological deposition upon aging. By elucidating the mechanism of Aβ metabolism, we intend to establish a new approach to prevent AD development by reducing Aβ burdens in aging brains. The other objective is to define the roles of intracellular proteases, calpains and caspases, and also of autophagy in the processes of neuronal dysfunction and degeneration in AD and other neurodegenerative diseases. Because these processes are relatively down-stream to Aβ deposition in the disease cascade, we expect the outcome to contribute to AD research in therapeutic rather than preventive terms. We also aim to identify the mechanisms (pathways), by which Aβ amyloidosis causes tauopathy and neurodegeneration. For this purpose, we generated 2nd generation mouse models of AD, which overproduce Aβ42 without overexpressing amyloid precursor protein. These models will also be useful for the search of biomarkers.
Main Research Fields
Biological Sciences
Related Research Fields
Biology / Medicine, Dentistry & Pharmacy
Keywords
- Alzheimer’s disease
- amyloid
- tau
- neuroinflammation
- neurodegeneration
Selected Publications
- Sasaguri, H., Nagata, K., Sekiguchi, M., Fujioka, R., Matsuba, Y., Hashimoto, S., Sato, K., Kurup, D., Yokota, T., Saido, T.C.
"Introduction of pathogenic mutations into the mouse Psen1 gene by Base Editor and Target-AID. "
Nat. Commun., 9(1):2282. (2018)
10.1038/s41467-018-05262-w - Nagata, K., Takahashi, M., Matsuba, Y., Okuyama-Uchimura, F., Sato, K., Hashimoto, S., Saito, T., Saido, T.C.
"Generation of single App knock-in mice reveals deletion mutations protective against the Alzheimer’s disease-like pathology."
Nat. Commun., 9(1) :1800. (2018)
doi: 10.1038/s41467-018-04238-0 - Sasaguri, H., Nilsson, P., Hashimoto, S., Nagata, K., Saito, T., De Strooper, B., Hardy, J., Vassar, R., Winblad, B., Saido, T.C.
"APP mouse models for Alzheimer’s disease preclinical studies."
EMBO J, 36, 2473-2787(2017).
10.15252/embj.201797397 - Saito, T., Matsuba, Y., Mihira, N., Takano, J., Nilsson, P., Itohara, S., Iwata, N., Saido, T.C.:
"Single App knock-in mouse models of Alzheimer’s disease."
Nat. Neurosci., 17, 661-663. (2014)
10.1038/nn.3697 - Nilsson, P., Loganathan, K., Sekiguchi, M., Matsuba, Y., Hui, K., Tsubuki, S., Tanaka, M., Iwata, N., Saito, T., Saido, T.C.:
"Aβ secretion and plaque formation depend on autophagy."
Cell Reports, 5(19), 61-69, doi: 10.1016/j.celrep.2013.08.042. (2013)
10.1016/j.celrep.2013.08.042 - Kakiya, N., Saito, T., Nilsson, P., Matsuba, Y., Tsubuki, S., Takei, N., Nawa, H., Saido T.C.:
"Cell-surface expression of the major Aβ degrading enzyme, neprilysin, depends on phosphorylation by MEK and dephosphorylation by protein phosphatase 1a."
J. Biol. Chem., 2 (2012).
10.1074/jbc.M112.340372 - Saito, T., Suemoto, T., Brouwers, N., Sleegers, K., Funamoto, S., Mihira, N., Matsuba, Y., Yamada, K., Nilsson, P., Takano, J., Nishimura, M., Iwata, N., Van Broeckhoven, C., Ihara, Y., Saido, T.C.:
"Potent amyloidogenicity and pathogenicity of Aβ43."
Nat. Neurosci.,14, 1023-1032. (2011)
10.1038/nn.2858 - Saito, T., Iwata, N., Tsubuki, S., Takaki, Y., Takano, J., Huang, S.-H., Suemoto, T., Higuchi, M., Saido, T.C.:
"Somatostatin regulates brain amyloid β peptide, Aβ42, through modulation of proteolytic degradation."
Nature Med., 11, 434-439.(2005)
10.1038/nm1206 - Higuchi, M., Iwata, N., Matsuba, Y., Sato, K., Sasamoto, K., Saido T.C.:.
"19F- and 1H-MRI detection of amyloid-β peptide in vivo."
Nature Neurosci., 8, 527-533. (2005)
10.1038/nn1422 - Iwata, N., Tsubuki, S., Takaki, Y., Shirotani, K., Lu, B., Gerard, N.P., Gerard, C., Hama, E., Lee, H.-J., Saido, T.C.:
"Metabolic regulation of brain Aβ by neprilysin."
Science, 292, 1550-1552. (2001)
10.1126/science.1059946
News & Media
-
-
-
Mouse model to explore links between Alzheimer’s disease and vascular amyloid plaques
-
-
-
-
RRH: New, relatively cheap potential treatment for Alzheimer’s disease
-
-
-
-
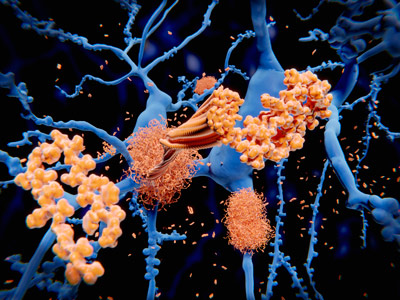
Missing link in late-onset Alzheimer’s disease
-
-
-

-
Potential new treatment for Alzheimer’s disease would be relatively cheap
-
-
-
-
New mouse model mimics earliest stages of Alzheimer’s disease
-
-
-
-
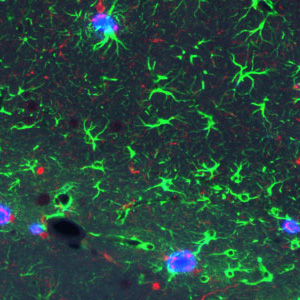
Alzheimer’s disease protein links plaques to cell death in mice
-
-
-
-
Mutation discovered to protect against Alzheimer’s disease in mice
-
Lab Members
Principal investigator
- Takaomi Saido
- Laboratory Head
Core members
- Takashi Saito
- Deputy Laboratory Head
- Naomasa Kakiya
- Research Scientist
- Kenichi Nagata
- Research Scientist
- Hiroki Sasaguri
- Research Scientist
- Shoko Hashimoto
- Special Postdoctoral Researcher
- Satoshi Tsubuki
- Research Specialist
- Maho Morishima
- Research Consultant
- Naoto Watamura
- Junior Research Associate
- Sho Yoshimatsu
- Junior Research Associate
- Naomi Mihira
- Technical Staff I
- Misaki Sekimoto
- Technical Staff I
- Yukiko Nagai
- Technical Staff I
- Yukio Matsuba
- Technical Staff I
- Ryo Fujioka
- Technical Staff I
- Naoko Kamano
- Technical Staff I
- Mika Takahashi
- Technical Staff I